DCAT Research & Benchmarking: Managing Business Continuity Risks Webinar
Robbinsville, New Jersey April 27, 2021 – the DCAT Research and Benchmarking Committee offers an annual survey which provides peer-to-peer insight for the bio/pharmaceutical manufacturing value chain. The Committee’s latest study, Managing Business Continuity Risks, is now available for download to all employees of DCAT Member Companies.
Jim Miller, DCAT Content Advisor/Consultant, recently moderated a panel discussion to review the report and results with the following DCAT Research and Benchmarking Committee members:
Christine Fuerst
Senior Director, Key Account Management Europe
Vetter Pharma International
David McCarthy
Senior Director, Sourcing and Enterprise Services
Pfizer
Vince Ricevuto
Vice President, Directs and Manufacturing Sites Procurement
John Ross
President
Mayne Pharma
Ken Seufert
CEO
JRS Pharma
Pnina Weitz
Global Head of Venture Capital Business Development & Relationship Management
Lonza
Overall, most companies had somewhat effective Business Continuity Plans (BCPs) in place prior to the COVID-19 pandemic.
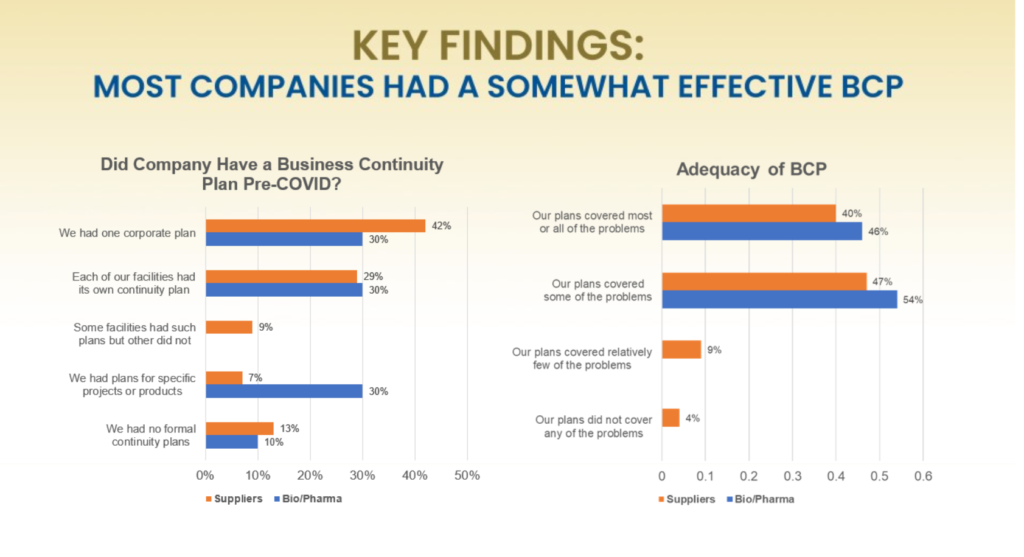
Most companies surveyed monitor a broad array of events, but while COVID-19 is a global pandemic, most of these monitored events are local or supplier-specific. According to the Committee members, companies must continue to challenge the industry beyond the pandemic by collaborating, enhancing supplier/management relationships, increasing cyber security, and standardizing dual sourcing.
Registrants can view a recording of this program on-demand, log in to access here.
About DCAT
The Drug, Chemical & Associated Technologies Association (DCAT) is a not-for-profit, global business development association whose unique membership model integrates both innovator and generic drug manufacturers and suppliers of ingredients, development and manufacturing services, and related technologies. We are committed to provide programs, events and services that help our members meet their business objectives, expand their network of customers and suppliers, and gain insight into industry trends, markets, and those issues impacting pharmaceutical development and manufacturing. With over 400 corporate members, DCAT is headquartered in Robbinsville, New Jersey.
Contact:
Carol Lee
DCAT Executive Director
+1.856.388.2963
clee@dcat.org

